The documentation in various systematic reviews and meta-analyses of selenium and cancer studies shows a significant inverse association between selenium intake and/or plasma/serum selenium status and cancer [Lee; Hurst; Cai].
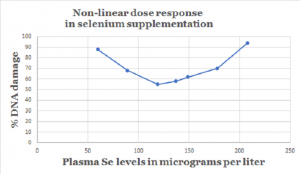
There is some evidence of a U-shaped relationship between plasma/serum selenium status and protection against cancer [Hurst; Rayman]. Low plasma/serum selenium status clearly correlates with higher risk of cancer. High plasma/serum selenium status correlates with no increased protective effect against cancer. The key is to find the supplement doses and subsequent plasma/serum status that give the best protection in between the two extremes.
Evaluating the evidence from published studies is complicated. We need to remember that the following factors affect the relationship between selenium status and/or intake and cancer risk:
- the form of the selenium supplement
- the baseline dietary selenium intakes
- the baseline plasma/serum selenium status
- the risk level of the study participants
- the form of the cancer
The form of the selenium supplement and cancer risk
Based on the evidence from randomized controlled trials, the effective form of selenium supplement for the prevention of cancer is the organic high-selenium yeast form [Clark, Yu]. Effective high-selenium yeast preparations such as the ones used in the PRECISE studies contain organic l-selenomethionine, Se-methylselenocysteine (=the compound found in garlic), and close to 30 other species of selenium [Larsen 2004].
Please note that the high-selenium yeast preparations contain an organic form of l-selenomethionine, which is the form found naturally in Brazil nuts, cereal grains, and legumes. The l-selenomethionine in high-selenium yeast preparations is not the synthetic l-selenomethionine used in the SELECT study [Lippman, Klein].
High-selenium yeast supplements are the comparatively very much safer selenium supplements [Schrauzer 2006].
Baseline selenium levels and cancer risk
All things considered, studies investigating the association between baseline plasma or serum selenium concentrations and the risk of cancer are preferred over studies investigating the association between baseline selenium intakes and the risk of cancer.
There are two reasons:
- Blood selenium levels can be measured more precisely than dietary selenium intakes can be measured because most of the data for dietary selenium intakes comes from study participants’ self-reports:
- data from food frequency questionnaires
- data from dietary history questionnaires
- data from supplement use questionnaires
- Even when the study participants report eating the same foods in the same quantities, there can be regional variations in the selenium content in the soil and in the food. Eight ounces of rice from one region may well contain less selenium than eight ounces of rice from a different region. (Note: eight ounces = ca. 227 grams)
Selenium supplementation and different forms of cancer
Dr. Cai and colleagues, in a 2016 meta-analysis of selenium exposure and cancer risk, concluded that higher selenium exposure is associated with decreased risk of the following specific types of cancer:
- breast cancer
- esophageal cancer
- gastric cancer
- lung cancer
- prostate cancer
However, higher selenium exposure has not yet been associated unequivocally with decreased risk of bladder cancer, colo-rectal cancer, or skin cancer [Cai].
Dose response to selenium and cancer risk
A once bandied about rule of thumb held that there would be approximately a 10% decrease in the risk of cancer for every increase of 10 micrograms per liter in plasma/serum selenium concentrations [Hurst].
That rule of thumb needs to be supported by more empirical evidence, and, remember: it may apply only within the boundaries of the U-shaped curve. The lower and upper boundaries of the effectiveness of selenium supplements against the development of cancer also need to be established more precisely.
Optimal plasma/serum selenium level and risk of cancer
The data from the Nutritional Prevention of Cancer study conducted in the United States by Professor Larry Clark and his colleagues indicated that the protective effect of selenium against the development of some forms of cancer began to be noticeable at a lower boundary of 120 micrograms of selenium per liter of plasma [Clark].
Dr. Hurst and colleagues in the United Kingdom completed a meta-analysis of studies of selenium status and the risk of prostate cancer. The data from their meta-analysis indicated that there was a gradual decreased risk of total prostate cancer and advanced prostate cancer in the range from 60 micrograms of selenium per liter of plasma up to 170 micrograms per liter.
At levels above 170 micrograms per liter, then, the protective effect of selenium supplementation flattened out, and more selenium ceased to be more protective. In other words, selenium supplementation does not confer the same protection in selenium-replete individuals as it does in selenium-deficient individuals [Hurst].
Differences between the NPC study results and the SELECT study results
There were two big differences in the study design of the Nutritional Prevention of Cancer study [Clark] and the study design of the SELECT study [Lippman; Klein]:
- The form of the supplement: organic high-selenium yeast in the NPC study versus synthetic l-selenomethionine in the SELECT study
- The median baseline serum selenium status of the study participants: 113 micrograms per liter in the NPC study and 135 micrograms per liter in the SELECT study [Lippman]
These two differences — the form of the selenium supplement and the baseline serum selenium status — may explain why the NPC study showed significant reductions in the risk of cancer, and the SELECT study did not.
Other factors affecting selenium supplements and risk of cancer
In a future article, I will want to address other factors that may affect the protective effect of selenium supplements against cancer:
- age
- gender
- optimal activity levels of various selenoproteins
- race
- smoking/non-smoking
At present, the scientific evidence summarized above points to the superiority of high-selenium yeast preparations for cancer prevention.
Sources
Cai, X., Wang, C., Yu, W., Fan, W., Wang, S., Shen, N., & … Wang, F. (2016). Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Scientific Reports, 619213. doi:10.1038/srep19213
Chiang, E. C., Shuren, S., Kengeri, S. S., Xu, H., Combs, Jr., G. F., Morris, J. S., Bostwick, D. G., & Waters, D. J. (2010). Defining the Optimal Selenium Dose for Prostate Cancer Risk Reduction: Insights from the U-Shaped Relationship between Selenium Status, DNA Damage, and Apoptosis. Dose Response, 8(3): 285–300. doi: 10.2203/dose-response.09-036.
Clark, L. C., Combs, G. J., Turnbull, B. W., Slate, E. H., Chalker, D. K., Chow, J., & Taylor, J. R. (1996). Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA, 276(24), 1957-1963.
Hurst, R., Hooper, L., Norat, T., Lau, R., Aune, D., Greenwood, D. C., & Fairweather-Tait, S. J. (2012). Selenium and prostate cancer: systematic review and meta-analysis. The American Journal of Clinical Nutrition, 96(1), 111-122. doi:10.3945/ajcn.111.033373
Klein, Eric A, Ian M, Jr Thompson, Catherine M Tangen, John J Crowley, M Scott Lucia, Phyllis J Goodman, and Laurence H Baker, et al. 2011. “Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT).” JAMA 306, no. 14: 1549-1556. MEDLINE with Full Text, EBSCOhost (accessed December 5, 2017).
Larsen, E. H., Hansen, M., Paulin, H., Moesgaard, S., Reid, M., & Rayman, M. (2004). Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. Journal of AOAC International, 87(1), 225-232.
Lee, E., Myung, S., Jeon, Y., Kim, Y., Chang, Y. J., Ju, W., & … Huh, B. Y. (2011). Effects of selenium supplements on cancer prevention: meta-analysis of randomized controlled trials. Nutrition and Cancer, 63(8), 1185-1195. doi:10.1080/01635581.2011.607544
Lippman, S. M., Klein, E. A., Goodman, P. J., Lucia, M. S., Thompson, I. M., Ford, L. G., & Coltman, C. J. (2009). Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 301(1), 39-51. doi:10.1001/jama.2008.864
Rayman, M. P. (2012). Selenium and human health. Lancet (London, England), 379(9822), 1256-1268. doi:10.1016/S0140-6736(11)61452-9.
Schrauzer, G. N. (2006). Selenium yeast: Composition, quality, analysis, and safety. Pure Appl. Chem., 78(1), 105–109. doi:10.1351/pac200678010105
Yu, S. Y., Zhu, Y. J., & Li, W. G. (1997). Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biological Trace Element Research, 56(1), 117-124.
The information presented in this review article is not intended as medical advice and should be used as such.
